abbott point of care covid test
Complete an application Form CMS-116 available on the CMS CLIA website or from a local State Agency. The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that delivers results within minutes allowing healthcare professionals to.
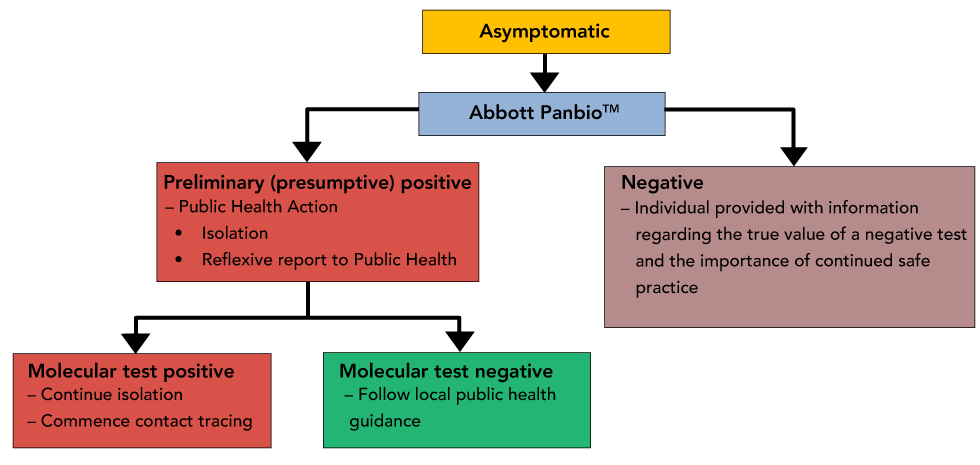
Interim Guidance On The Use Of Abbott Panbio Covid 19 Antigen Rapid Test Ccdr 47 1 Canada Ca
Globalpointofcareabbott の BinaxNOW COVID-19 Antigen Self-Test Abbott Point of Care の解説文やアートワーク歌詞の他にもテイストが似ているアーティストを掲載しています.

. The company says it will ramp up its. Abbott to market starting next week a fast point-of-care coronavirus test delivering positive results in 5min and negative results in 13min. Abbotts molecular point-of-care test for COVID-19 delivers positive results in as little as five minues and negative results in 13 minutes.
Our Rapid COVID-19 Tests Our BinaxNOW test is the size of a credit card and requires no specialized instrumentation. Abbotts latest COVID-19 product isnt the first point-of-care test on the US. Abbotts BinaxNOW COVID-19 Ag Card test can identify these antigens which are typically detected after symptoms start.
The ID NOW platform combines the benefits of speed and accuracy for the fastest molecular results in the market. Abbott received emergency use authorization EUA from the US. Abbott Laboratories ID NOW COVID-19 point-of-care test will be shipped to hospitals care clinics and doctors offices across the country starting Wednesday.
ID NOW is an FDA approved CLIA-waived instrument which means that. ABBOTT ID Now COVID-19 POINT OF CARE TESTING 5192020 The following guidance is for institutions that have an Abbott ID NOW instrument and test kits for performing CLIA-waived rapid point of care COVID-19 testing. Diagnostics Testing May 27 2020.
Allocation and distribution of instruments and test kits will be determined by Central. Danaher molecular diagnostics subsidiary Cepheid claimed that milestone with an EUA awarded March 20. Abbott Laboratories ABT announced the receipt of the FDAs Emergency Use Authorization EUA for its molecular point-of-care test ID NOW COVID-19 for the detection of the novel coronavirus.
Food and Drug Administration FDA for the fastest available molecular point-of-care test for the detection of novel coronavirus COVID-19 delivering positive results in as little as five minutes and negative results in 13 minutes. Abbott has received emergency use authorization EUA from the US. The ID NOW COVID-19 test returns positive results in 13 minutes or less to enable immediate clinical decisions during the first patient visit.
The tests are intended to identify the virus by recognizing a unique section of the coronavirus genome and amplifying that portion until theres enough for. Detects active COVID-19 infection. It can be obtained as follows.
The tests can be used in point-of-care settings and at home with an online service provided by eMed. Ad Making medical supply ordering easy. Food and Drug Administration Emergency Use Authorization EUA.
Get results in 15 minutes. NAVICA displays results from the 15-minute Abbott BinaxNOW COVID-19 Ag Card rapid antigen test to help individuals make informed decisions. Results from the simple nasal swab are available in 15 minutes through testing individuals suspected of COVID-19.
Our ID NOW test for COVID-19 is the fastest molecular point-of-care rapid test available today and has been delivering reliable results when and where theyre needed. The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that delivers results within minutes allowing healthcare professionals to make clinical decisions during a patient visit. Abbotts rapid tests are among the most widely-used in the US with more than 200 million of our BinaxNOW and ID NOW rapid tests used in urgent care clinics doctors offices pharmacies nursing homes and schools since April 2020.
The revolutionary NAVICA app helps people navigate daily life in a new normal. But the turnaround time and reach of Abbotts new test so far appears unparalleled in the US. This joins Abbotts RealTime SARS-CoV-2 test which was approved under a EUA earlier this month as well as a growing list of companies whose diagnostic tests are being.
Will deliver 50K testsday to start. Abbott has rapid point-of-care solutions to support your COVID-19 and influenza testing needs ID NOW COVID-19 The ID NOW COVID-19 assay is now available for use on the ID NOW platform under US. Download the BinaxNOW COVID-19 Antigen Self Test Product Insert.
Capture your results in the NAVICA app for self reporting. Abbott s new point-of-care test for the novel coronavirus that causes COVID-19 was approved by the US. Abbott Laboratories ID NOW COVID-19 point-of-care test will be shipped to hospitals care clinics and doctors offices across the country starting Wednesday.
BinaxNOW COVID-19 Ag Card has received US. The Abbott ID NOW COVID-19 test brings rapid testing to a wide range of front-line healthcare environments such as physicians offices urgent care clinics and hospital emergency departments. Food and Drug Administration Emergency Use Authorization EUA.
Abbott to market starting next week a fast point-of-care coronavirus test delivering positive results in 5min and negative results. The company says it will ramp up its. Food and Drug Administration FDA for the ID NOW COVID-19 test in March 2020.
The ID NOW COVID-19 test is a rapid molecular point-of-care test that detects COVID-19 in 13 minutes or less. The companys share price increased 64 to close at 7934 on Mar 30 since the news came on Mar 27. Abbott Launches Molecular Point-of-Care Test to Detect Novel Coronavirus in as Little as Five Minutes - The Abbott ID NOW COVID-19 test brings rapid testing to the front lines.
The availability and ease-of-access of ID NOW which delivers results in minutes rather than a day or more is helping to reduce the spread and risk of infection by. Bttn is the faster smarter and less expensive solution to medical supplies. The easy to use ID NOW platform is designed for near-patient point-of-care use.
It is used on our ID NOW platform. Save when you order supplies from bttn. A simple solution for COVID-19 infection detection with rapid results in the convenience of home.
According to Abbott the rapid test which runs on the ID NOW platform is an. What makes this test so different is where it can be used. Food and Drug Administration FDA under Emergency Use Authorization EUA.
Cepheid said there are about 5000 of the systems that process its. A CLIA certificate is required to perform point-of-care testing. A CLIA Certificate of Waiver is appropriate if SARS-CoV-2 point-of-care testing is the only testing being performed.
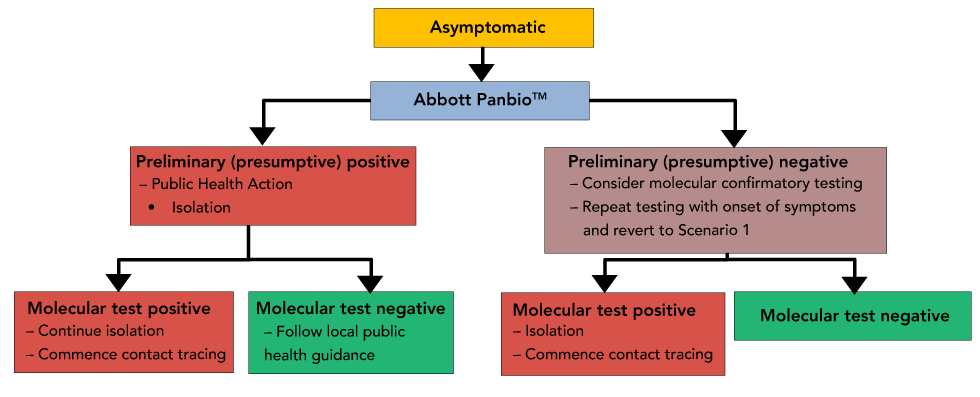
Interim Guidance On The Use Of Abbott Panbio Covid 19 Antigen Rapid Test Ccdr 47 1 Canada Ca

Abbott Id Now Covid 19 Instructions Modified

Panbio Sars Cov 2 Igg Elisa Abbott Point Of Care

This Is How The New Abbott Labs Covid 19 Rapid Test Works Youtube

Clinical Performance Of Id Now In Individuals With Compatible Sars Cov 2 Symptoms In Walk In Centres Accelerated Turnaround Time For Contact Tracing Ccdr 47 12 Canada Ca

Rapid Testing To Expand Return Of Mass Vaccination Sites For Covid Booster Shots In B C Vernon Morning Star

Id Now Training Videos Abbott Point Of Care

Virus News Abbott Launches 5 Minute Covid 19 Test Bloomberg
Aacc Guidance Document On Management Of Point Of Care Testing Aacc Org

Navica For Individuals Abbott Point Of Care

Navica For Individuals Abbott Point Of Care

Abbott S Istat Comes Out On Top In Study Comparing Blood Clot Monitoring Tests 360dx

Navica For Individuals Abbott Point Of Care
Abbott Destroyed Millions Of Covid Test Cards When Demand Plummeted Now It S Scrambling To Ramp Back Up Fierce Biotech
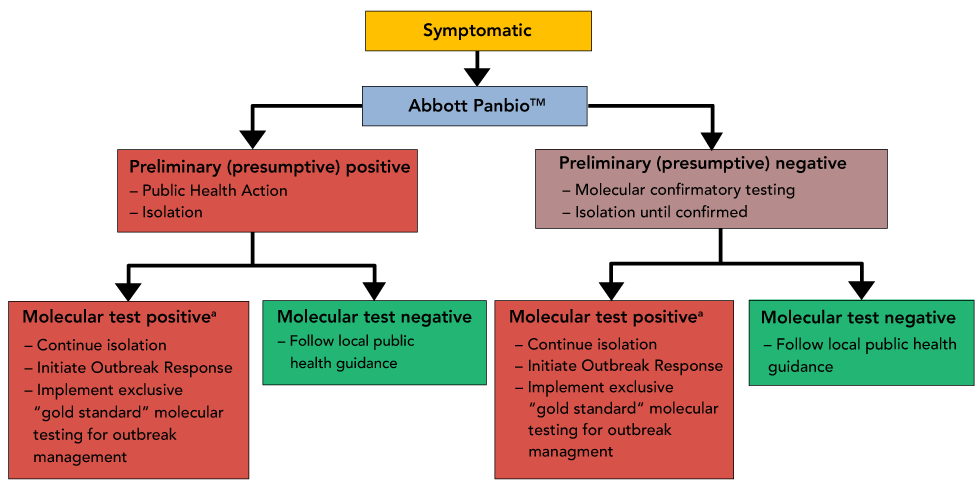
Interim Guidance On The Use Of Abbott Panbio Covid 19 Antigen Rapid Test Ccdr 47 1 Canada Ca
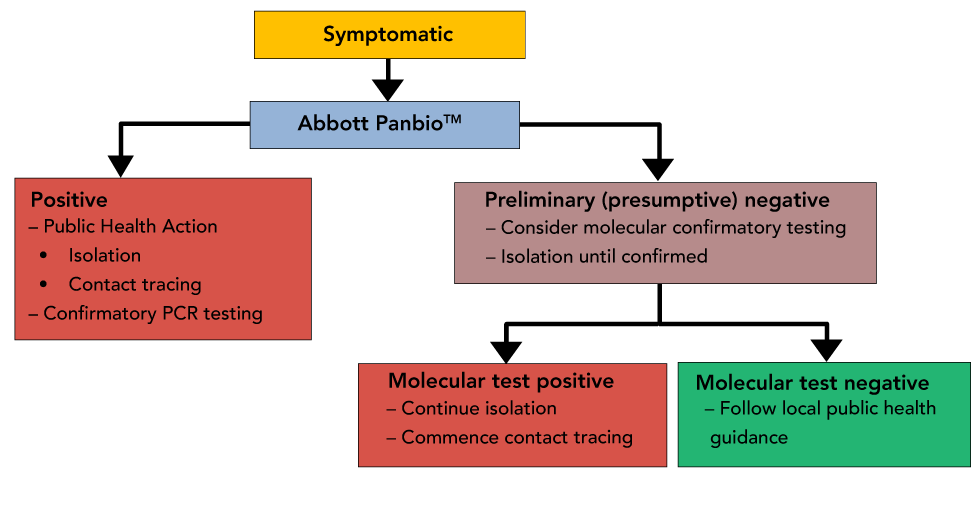
Interim Guidance On The Use Of Abbott Panbio Covid 19 Antigen Rapid Test Ccdr 47 1 Canada Ca
Virus News Abbott Launches 5 Minute Covid 19 Test Bloomberg

Interim Guidance On The Use Of Abbott Panbio Covid 19 Antigen Rapid Test Ccdr 47 1 Canada Ca